Describe How Dilution Was Used to Prepare the Starch Solutions
When the beaker cools down we need to filter the solution. Prepare the albumin solution.

Benedict S Test Reagent Preparation Principle Procedure Reaction
Remove the outer cap and dropper cap from the plastic bottle labeled Potato Starch b.
. M dilution V dilution M stock V stock. 2 Iodine solution Iodine is only sparingly soluble in water. Water is added to 050 L of a 12 M NaOH solution to make 30 L of a diluted NaOH solution.
A 100-mL sample of a 25 mv KOH solution is diluted with water so that the final volume is. Then set up a number of tubes containing 9 ml each of broth alone for dilutions. Measure the volume V 1 of the solution with concentration C 1.
The dilution factor can be used to calculate the volumes. Pour the starch solution through the filter paper and funnel and. State whether the concentration of a solution is directly or indirectly proportional to its volume.
If a different molarity is. The easiest method is to make a series of 1 in 10. When you know all four values in the equation C 1 V 1 C 2 V 2 perform your dilution as follows.
To do this take a funnel and place a filter paper inside it. Prepare the potato starch solution. A serial dilution is a series of sequential dilutions used to reduce a dense culture of cells to a more usable concentration.
This undiluted tube will be our tube 1. Begin with 4 clean tubes Label three tubes A for the amylase reaction and one tubes C for the control. When starch is heated in water decomposition occurs and beta-amylose.
Write the dilution equation. C 1 V 1 C 2 V 2 where. To make a fixed amount of a dilute solution from a stock solution you can use the formula.
You can identify a dilution solution by the amount of solute in the total volume expressed as a. Remove the outer cap and plastic dropper cap from the bottle labeled Albumin Powder b. 10 M 50 ml 20 M x ml x 10 M 50 ml20.
You have series of starch solutions with known concentrations that you have prepared by serial dilution and which you measure using Lugols. Add a small volume of distilled deionized water to dissolve the salt. Post that place the beaker aside to let it cool.
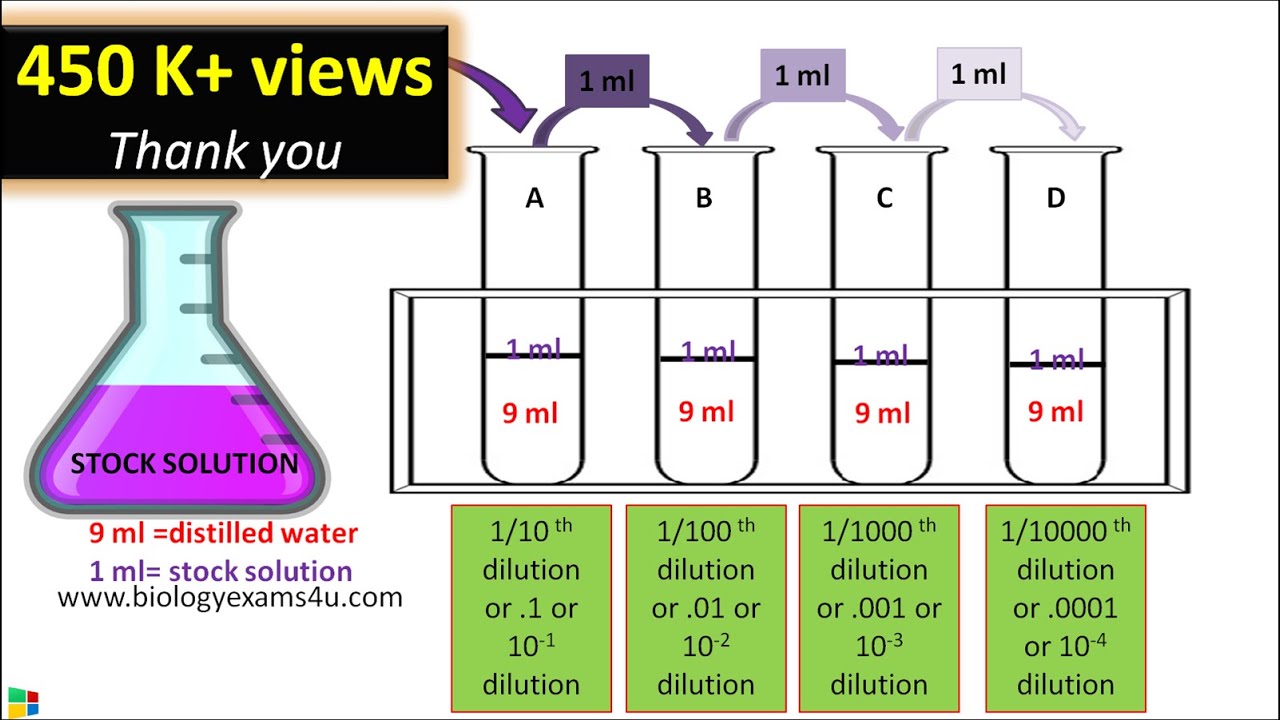
Biology questions and answers. Add distilled water to the bottle until it is filled to the neck and. This provides an initial dilution of 10 -1.
Starch is a viable indicator in the titration process because it turns deep dark blue when iodine is present in a solution. Add 01 mL of starch solution 1gL to each tube. The pipette tip is discarded and a new pipette tip.
Using C 1 V 1 C 2 V 2. Typically the dilution factor remains constant for each dilution resulting in an exponential decrease in concentration. To prepare a liter of a simple molar solution from a dry reagent Multiply the formula weight or MW by the desired molarity to determine how many grams of reagent to use.
Boil the water and starch mixture for about 10 mins. For example a ten-fold serial dilution could result in the following. Serial dilutions involve diluting a stock or standard solution multiple times in a row.
Add distilled water to the bottle until it is filled to the neck and. These two components proportionally combine to create a dilution. Fill the flask to the 1 L line.
1 Starch solution Add a weighed amount of starch 05 g or 10 g to a little heated water mix to a paste then dilute to 50 or 100 cm 3. The dilution factor is obtained from the initial concentration of the stock solution and the fmal concentration of the diluted solution. Standard Curve Data Dilutions A 590 Concentration D1 D2 D3 D4 D5 D6 D7 B.
Draw 1 mL of undiluted solution from test tube US with a pipette and transfer it to the test tube labeled 110 containing 9 mL of the dilution liquid and. The following is a brief explanation of some ways of calculating dilutions that are common in biological science and often used at Quansys Biosciences. Take 1 ml from the first tube and add it to the 9 ml of plain broth you.
The dilution is thoroughly mixed by emptying and filling the pipette several times. Place the NaCl in a 1-liter volumetric flask. Perform the first dilution.
Practice the Assay Procedure. V 1 Volume of stock solution needed to make the new solution. Your first step is to calculate the volume of stock solution that is required.

Solutions Part 2 Preparation Of Solutions Molar Normal And Dilution Labpedia Net

Serial Dilution Method Protocol Step Wise Explanation Youtube

Leaf Structure Lab Structure And Function Leaf Structure And Function Leaf Structure
No comments for "Describe How Dilution Was Used to Prepare the Starch Solutions"
Post a Comment